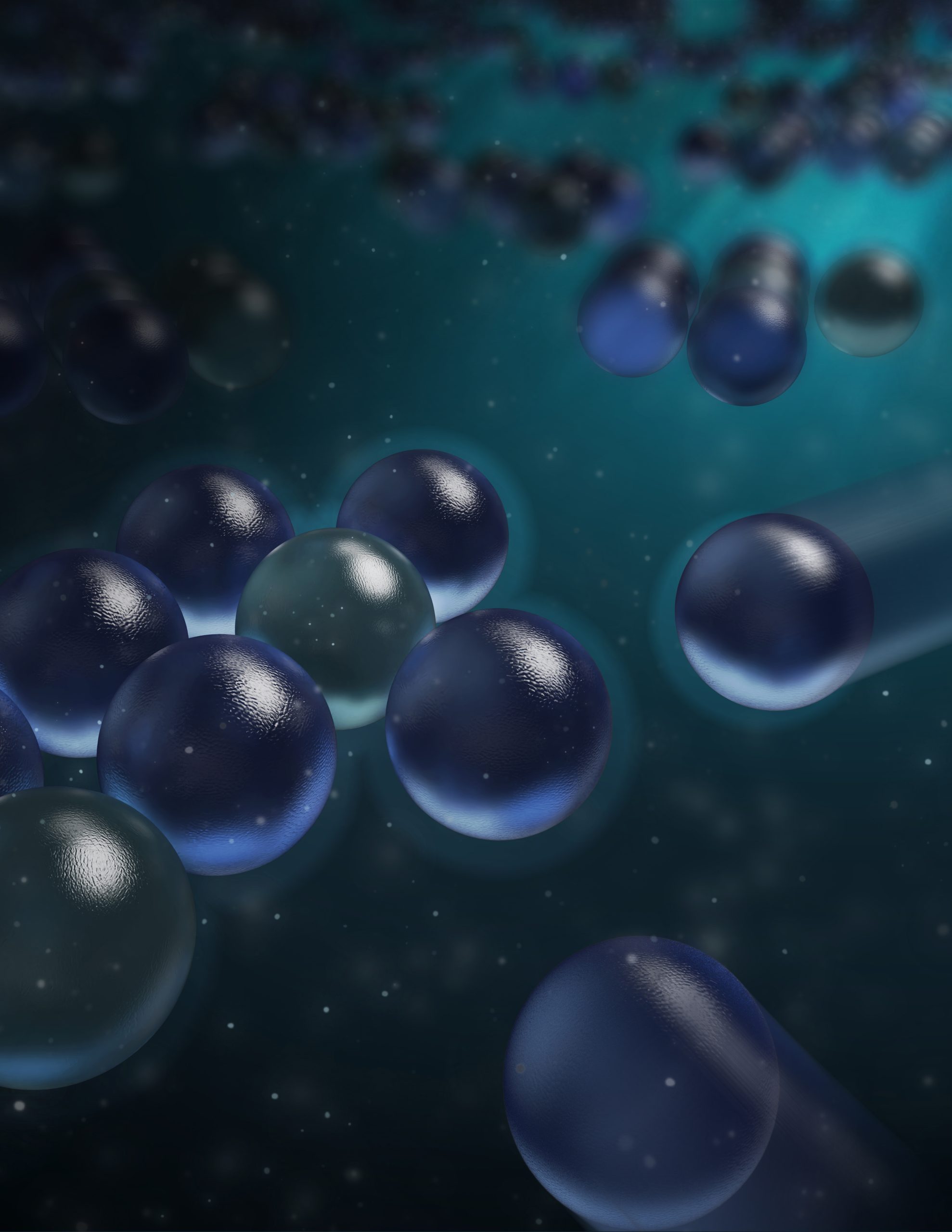
A new study has upended a fundamental principle of physics by showing that similarly charged particles can attract each other in solution, with the effect varying between positive and negative charges depending on the solvent. This discovery has important implications for various scientific processes, including self-assembly and crystallization. The research reveals the importance of solvent structure at the interface in determining interactions between particles, challenging long-held beliefs and pointing to the need to reevaluate our understanding of electromagnetic forces. Credit: Zhang Kang
“Opposite charges attract; “Like charges repel each other” is a fundamental principle of fundamental physics. However, a new study from the University of Oxford, recently published in the journal nature nanotechnology, He demonstrated that similarly charged particles in solution can actually attract each other over long distances.
Also surprisingly, the team found that the effect differs for positively and negatively charged particles, depending on the solvent.
Besides overturning long-held beliefs, these results have immediate implications for a range of processes involving intermolecular and intermolecular interactions across different length scales, including self-assembly, crystallization, and phase separation.
The team of researchers, based at the Department of Chemistry at the University of Oxford, found that negatively charged particles attract each other at large distances, while positively charged particles repel each other, while the opposite was the case for solvents such as alcohol.
These results are surprising because they seem to contradict the central electromagnetic principle that the force between charges of the same sign is repulsive at all separations.
Experimental observations
Now, using bright-field microscopy, the team has tracked tiny, negatively charged silica particles suspended in water, and found that the particles attract each other to form ordered, hexagonal clusters. However, the positively charged amino-silica molecules did not form clusters in water.
Using the theory of particle interactions that takes into account the structure of the solvent at the interface, the team demonstrated that for negatively charged particles in water, there is an attractive force that outweighs the electrostatic repulsion at large separation distances, leading to the formation of clumps. For positively charged particles in water, this solvent-driven reaction is always repulsive, and no aggregates form.
This effect was found to depend on pH: the team was able to control the formation (or non-formation) of clusters of negatively charged particles by changing the pH. Regardless of pH, positively charged molecules do not form clusters.
Solvent-specific effects and additional discoveries
Naturally, the team wondered whether it would be possible to switch the effect on charged particles, such that positively charged particles form clusters while negatively charged particles do not. By changing the solvent to alcohols, such as ethanol, which have a different interface behavior from water, this is exactly what they observed: the positively charged amino-silica molecules formed hexagonal groups, whereas the negatively charged silica did not.
According to the researchers, this study involves a fundamental recalibration in understanding that will impact the way we think about various processes such as the stability of pharmaceutical and fine chemical products or pathological dysfunction associated with molecular aggregation in human diseases. The new results also provide evidence of the ability to explore properties of the interfacial electrical potential generated by the solvent, such as its sign and size, which were previously thought to be unmeasurable.
“I am really very proud of my graduate students, as well as the undergraduate students, who have all worked together to move the needle on this fundamental discovery,” says Professor Madhavi Krishnan (Department of Chemistry, University of Oxford), who led the study.
“I still find it fascinating to see these particles attracting each other, even after I've seen it a thousand times,” says Sida Wang (Department of Chemistry, University of Oxford), first author of the study.
Reference: “A long-range charge-dependent force drives ad hoc assembly of matter in solution” by Syda Wang, Rowan Walker Gibbons, Bethany Watkins, Melissa Flynn, and Madhavi Krishnan, 30 February 2024, Nature nanotechnology.
doi: 10.1038/s41565-024-01621-5

“Amateur organizer. Wannabe beer evangelist. General web fan. Certified internet ninja. Avid reader.”



/cdn.vox-cdn.com/uploads/chorus_asset/file/25550621/voultar_snes2.jpg)

More Stories
Watch a Massive X-Class Solar Explosion From a Sunspot Facing Earth (Video)
New Study Challenges Mantle Oxidation Theory
The theory says that complex life on Earth may be much older than previously thought.