The researchers overturned traditional models of how water molecules behave on the surface of salt water, revealing new insights into the distribution and orientation of ions. This achievement, achieved through advanced technologies, has major implications for climate science and technology. Credit: SciTechDaily.com
Pioneering research shows that water molecules on the surface of salt water behave differently than previously thought, providing new perspectives for environmental science and technology.
Textbook models will need to be redrawn after a team of researchers discovered that water molecules on the surface of salt water are organized differently than previously thought.
Many important interactions related to climate and environmental processes occur when water molecules interact with air. For example, ocean evaporation plays an important role in atmospheric chemistry and climate science. Understanding these responses is critical to efforts to mitigate human impact on our planet.
The distribution of ions at the air-water interface can affect atmospheric processes. However, a precise understanding of the microscopic interactions at these important interfaces has been extensively discussed to date.
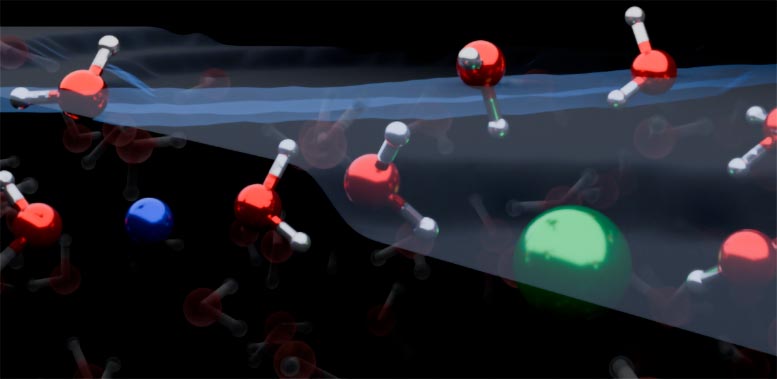
Graphical representation of the liquid/air interface in a sodium chloride solution. Credit: Yair Litman
Innovative search techniques
In a research published today (January 15) in the magazine Nature chemistryResearchers from the University of Cambridge and the Max Planck Institute for Polymer Research in Germany have shown that the ions and water molecules on the surface of most salt water solutions, known as electrolytes, are organized in a very different way than is traditionally understood. This could lead to better models of atmospheric chemistry and other applications.
The researchers set out to study how water molecules are affected by the distribution of ions at the specific point where air and water meet. Traditionally, this is done using a technique called Vibratory Frequency Sum Generation (VSFG). Using laser radiation technology, it is possible to directly measure molecular vibrations at these key interfaces. However, although it is possible to measure the strength of signals, this technique does not measure whether the signals are positive or negative, which has made the results difficult to interpret in the past. In addition, using experimental data alone can give ambiguous results.
The team overcame these challenges by using a more advanced form of VSFG, called heterodyne-detected (HD)-VSFG, to study different electrolyte solutions. They then developed advanced computer models to simulate the facades in different scenarios.
Revolutionizing traditional models
The combined results showed that both positively charged ions, called cations, and negatively charged ions, called anions, are depleted from the water/air interface. The cations and anions in simple electrolytes direct the water molecules in the up and down directions. This is the opposite of textbook models, which teach that ions form an electrical double layer and direct water molecules in only one direction.
Co-first author, Dr Yair Litman, from the Yosef Hamid Department of Chemistry, said: “Our work shows that the surface of simple electrolyte solutions has a different ionic distribution than previously thought and that the ion-enriched underlying surface determines how the interface is organized. At the top are a few layers of water.” Pure, then an ion-rich layer, and finally the bulk salt solution.
Co-first author Dr Kuo Yangqiang from the Max Planck Institute said: “This paper shows that the combination of high-level HD-VSFG and simulations is an invaluable tool that will contribute to the molecular-level understanding of liquid interfaces.”
Professor Mischa Poon, who heads the Department of Molecular Spectroscopy at the Max Planck Institute, added: “These types of interfaces are found everywhere on the planet, so studying them not only helps our basic understanding, but could also lead to better devices and technologies.” These same methods for studying solid/liquid interfaces, which could have potential applications in batteries and energy storage.
Reference: “Surface stratification determines the surface aqueous structure of simple electrolyte solutions” 15 January 2024, Nature chemistry.
doi: 10.1038/s41557-023-01416-6

“Amateur organizer. Wannabe beer evangelist. General web fan. Certified internet ninja. Avid reader.”



/cdn.vox-cdn.com/uploads/chorus_asset/file/25550621/voultar_snes2.jpg)


More Stories
Watch a Massive X-Class Solar Explosion From a Sunspot Facing Earth (Video)
New Study Challenges Mantle Oxidation Theory
The theory says that complex life on Earth may be much older than previously thought.