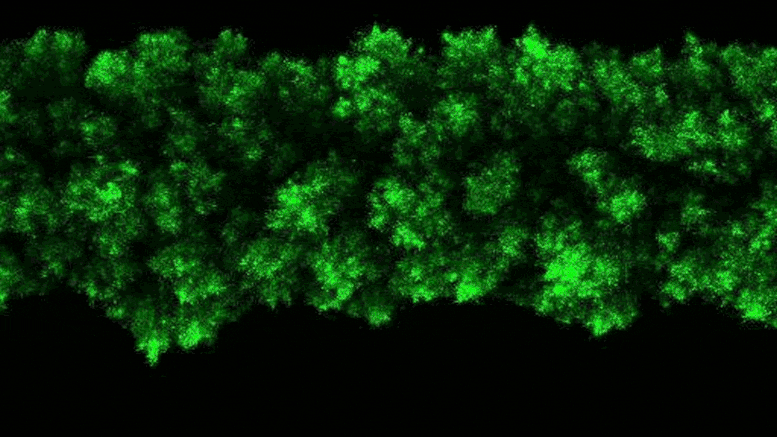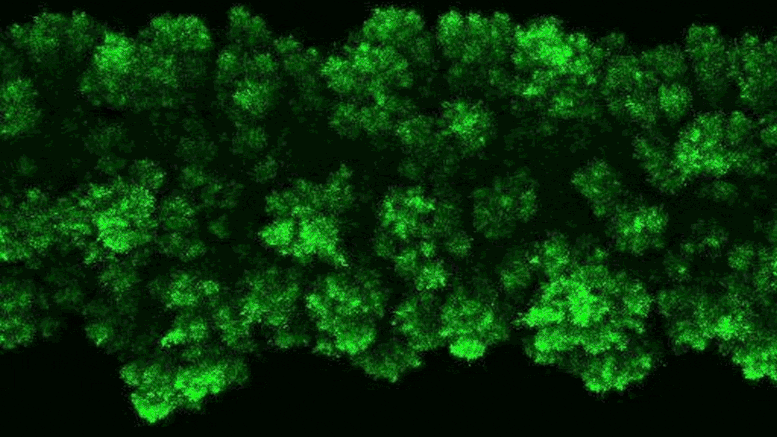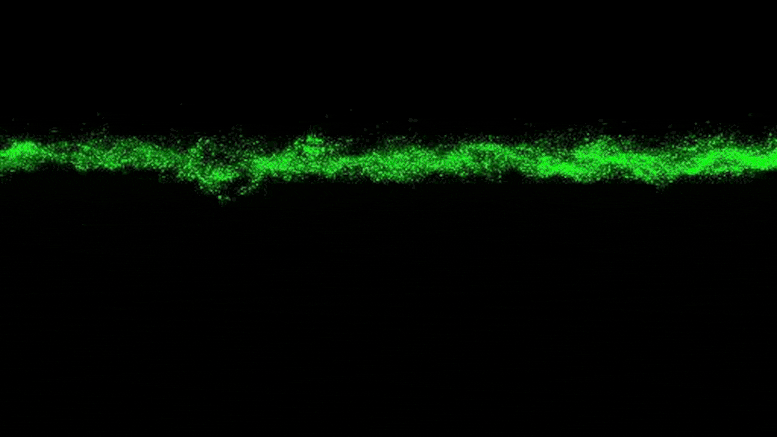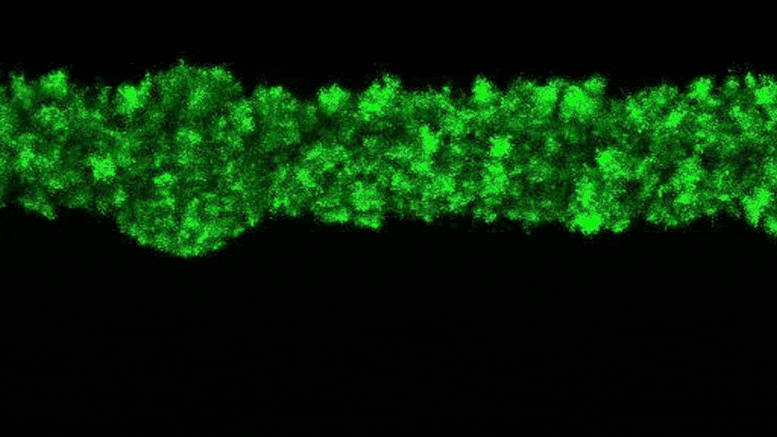
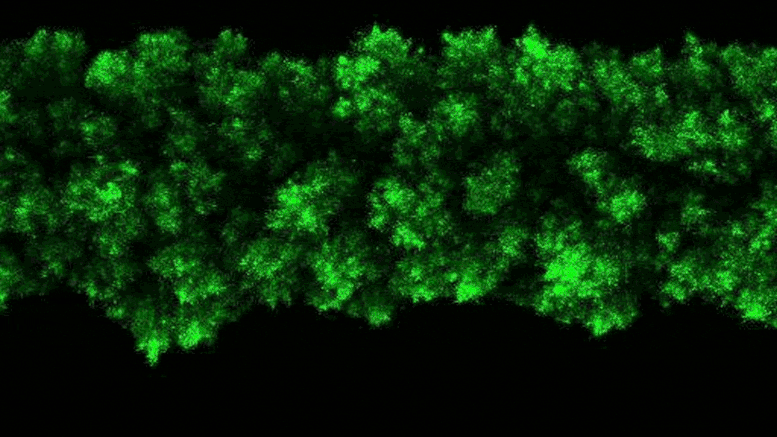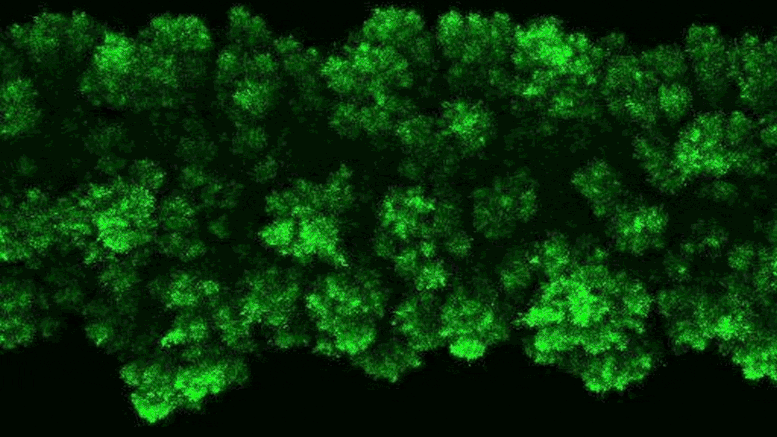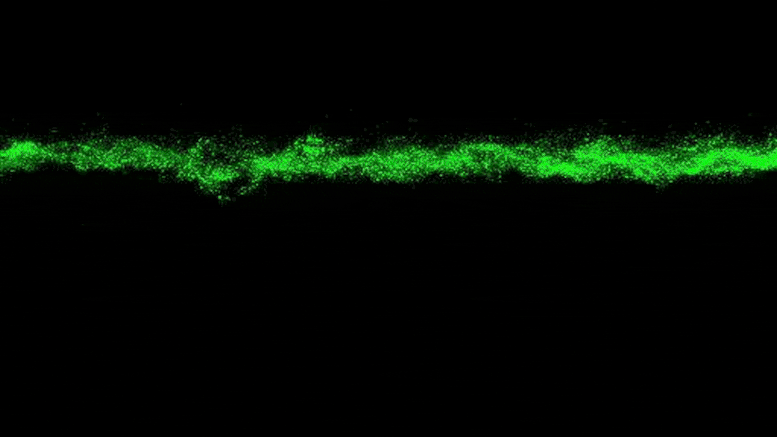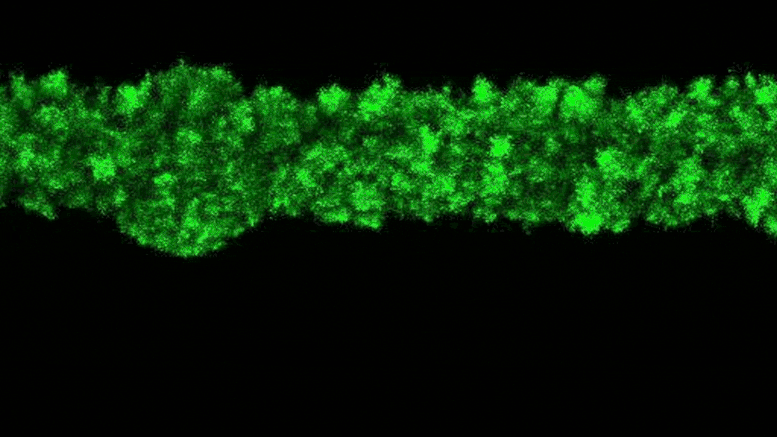
The researchers were able to observe the clumpy growth of the bacterial colonies in three dimensions. Credit: Neil Adelantar/Princeton University
The researchers found that colonies of bacteria are formed in three dimensions with rough, crystal-like shapes.
Bacterial colonies often grow in lines on Petri dishes in laboratories, but no one understood how colonies arrange themselves in more realistic 3D environments, such as tissues and gels in human bodies or soil and sediment in the environment, until now. This knowledge may be important for the advancement of environmental and medical research.
a Princeton University The team has now developed a way to monitor bacteria in 3D environments. They discovered that as bacteria grow, their colonies continually form wonderfully jagged shapes resembling a branched head of broccoli, far more complex than what we see in a petri dish.
“Since bacteria were discovered more than 300 years ago, most laboratory research has studied them in test tubes or on petri dishes,” said Sujit Datta, assistant professor of chemical and biological engineering at Princeton University and lead author of the study. This was the result of practical limitations rather than a lack of curiosity. “If you try to watch bacteria grow in tissue or in soil, these are opaque, and you can’t actually see what the colony is doing. That was the real challenge.”

The researchers are Sujit Datta, assistant professor of chemical and biological engineering, Alejandro Martínez Calvo, postdoctoral researcher, and Ana Hancock, graduate student in chemical and biological engineering. Credit: David Kelly Crowe of Princeton University
Data’s research group discovered this behavior using a pioneering experimental setup that enables them to make previously unheard-of observations of bacterial colonies in their natural, three-dimensional state. Unexpectedly, scientists have discovered that the growth of wild colonies consistently resembles other natural phenomena such as crystal growth or frost spreading on window glass.
“These types of jagged, branching shapes are ubiquitous in nature, but usually in the context of growing or clumping inanimate systems,” Datta said. “What we found is that growth in 3D bacterial colonies exhibit a very similar process despite the fact that these are groups of organisms.”
This new explanation of how colonies of bacteria evolve in three dimensions was recently published in the journal Proceedings of the National Academy of Sciences. Datta and his colleagues hope their discoveries will aid a wide range of bacterial growth research, from creating more effective antimicrobials to pharmaceutical, medical and environmental research, as well as procedures that harness bacteria for industrial use.
“At a fundamental level, we are excited that this work is revealing surprising connections between the development of form and function in biological systems and studies of non-living growth processes in materials science and statistical physics. But we also believe that this new insight into when and where cells grow in 3D will be of interest to any Someone interested in bacterial growth, such as environmental, industrial and biomedical applications,” Datta said.
For several years, Datta’s research team has been developing a system that allows them to analyze phenomena that would normally be hidden in opaque conditions, such as the flow of fluids through soil. The team uses specially designed hydrogels, which are water-absorbent polymers similar to those found in contact lenses and jellies, as matrices to support the growth of bacteria in 3D. Unlike those common versions of hydrogels, Data materials consist of very small spheres of hydrogel that are easily deformed by bacteria, allow free passage of oxygen and nutrients that support bacterial growth and are transparent to light.
“It’s like a ball pit where each ball is an individual hydrogel. It’s microscopic, so you can’t really see it,” said Datta. The research team calibrated the composition of the hydrogel to mimic the structure of soil or tissue. The hydrogel is strong enough to support bacterial colony growth without introducing resistance. sufficient to restrict growth.
“As bacterial colonies grow in the hydrogel matrix, they can easily rearrange the globules around themselves so that they are not trapped,” he said. “It’s like sinking your arm into a ball pit. If you pull it through, the balls rearrange themselves around your arm.”
The researchers experimented with four different types of bacteria (including one that helps create the pungent taste of kombucha) to see how they grew in three dimensions.
“We changed the cell types, the nutrient conditions, and the properties of the hydrogel,” Datta said. The researchers saw the same coarse growth patterns in every case. “We have systematically changed all of these parameters, but this seems to be a general phenomenon.”
Data said two factors appear to be causing cauliflower-shaped growths on the surface of the colony. First, bacteria with higher levels of nutrients or oxygen will grow and multiply faster than those in a less abundant environment. Even the most consistent environments have some uneven nutrient densities, and these differences cause spots in the surface of the colony to move forward or lag behind. This repeats in three dimensions, causing the colony of bacteria to form bumps and nodules as some subsets of bacteria grow more rapidly than their neighbors.
Second, the researchers note that in 3D growth, only bacteria near the surface of the colony grow and divide. The bacteria squashed in the center of the colony seem to descend into a hibernating state. Because the bacteria inside weren’t growing and dividing, the outside didn’t experience pressure that would cause it to expand evenly. Instead, its expansion is mainly driven by growth along the edge of the colony. The growth along the edge is subject to nutrient changes which eventually lead to stunted and erratic growth.
“If the growth was uniform, and there was no difference between the bacteria inside the colony and those on the periphery, it would be like filling a balloon,” said Alejandro Martínez Calvo, a postdoctoral researcher at Princeton University and first author of the paper. “The pressure from within will fill in any turmoil on the extremities.”
To explain why this stress was not present, the researchers added a fluorescent tag to proteins that become active in cells when bacteria grow. The fluorescent protein glows when the bacteria are active and remains dark when they are not. By observing the colonies, the researchers saw that the bacteria at the edge of the colony were bright green, while the core remained dark.
“The colony basically organizes itself into a core and a shell that behave in very different ways,” Datta said.
The theory, Datta said, is that the bacteria on the edges of the colony pick up most of the nutrients and oxygen, leaving little for the inner bacteria.
“We think they hibernate because they are hungry,” Datta said, though he cautioned that more research is needed to explore this.
Data said that experiments and mathematical models used by the researchers found that there was an upper limit to the ridges that formed on the colony’s surfaces. The bumpy surface is the result of random differences in oxygen and nutrients in the environment, but the randomness tends to be even within certain limits.
“The roughness has an upper limit to how big it can be – the size of a floret if we compare it to a broccoli,” he said. “We were able to predict this with mathematics, and it seems to be an inevitable feature of the growth of large colonies in 3D.”
Because bacterial growth tends to follow a similar pattern to crystal growth and other well-studied phenomena of non-living materials, Datta said the researchers were able to adapt standard mathematical models to reflect bacterial growth. He said future research will likely focus on better understanding the mechanisms behind growth, the implications for rough growth forms of colony functioning, and applying these lessons to other areas of concern.
“Ultimately, this work gives us more tools to understand, and ultimately to control, how bacteria grow in nature,” he said.
Reference: “Morphological instability and growth coarseness of three-dimensional bacterial colonies” by Alejandro Martínez-Calvo, Tapumoy Bhattacharjee, R Conan Pai, Hau Njie Lu, Anna M Hancock, Ned S. Wingreen and Sojit S-Data, Oct. 18, 2022, Available here. Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2208019119
The study was funded by the National Science Foundation, the New Jersey Health Foundation, the National Institutes of Health, the Eric and Wendy Schmidt Transformational Technology Fund, the Pew Medical Scientists Fund, and the Human Frontier Science Program.




/cdn.vox-cdn.com/uploads/chorus_asset/file/25550621/voultar_snes2.jpg)


More Stories
Watch a Massive X-Class Solar Explosion From a Sunspot Facing Earth (Video)
New Study Challenges Mantle Oxidation Theory
The theory says that complex life on Earth may be much older than previously thought.